Introduction
European Union (EU) Clinical Trials Regulation No 536/2014 (‘the Regulation’) has been operational since 31 January 2022. It covers interventional clinical trials of investigational medicinal products for human use and has an important impact on all aspects of trial set-up, operation and reporting in the EU and the European Economic Area (EEA).
This post summarises Year 2 (January 2023 to January 2024) of the Regulation from a sponsor perspective, with a focus on transparency aspects.
Purpose of Transition Period
There is a three-year transition period for the Regulation. During Year 1 of the transition period (31 January 2022 to 30 January 2023), new EU trials could follow the earlier EU Clinical Trials Directive (CTD) or the Regulation, with the following recommendations:
• New trials expected to be ongoing after 30 January 2025 were encouraged to follow the Regulation.
• Trials ongoing under the EU CTD had the option to transition to the Regulation.
• Voluntary Harmonisation Procedure trials had to follow the Regulation or file national submissions for substantial amendments under the EU CTD.
A summary of the major developments during Year 1 is available here.
From Year 2 onwards, the following rules applied:
• All new trials had to be submitted under the Regulation; submission of new trials under the EU CTD was no longer possible.
• For ongoing EU CTD trials, addition of new member states was no longer possible; such trials had to first be transitioned to the Regulation; an additional member state could be added once the trial had been updated with a substantial modification.
In addition, during Year 2, sponsors were expected to begin moving EU CTD trials anticipated to be ongoing after 30 January 2025 to the Regulation.
Developments in Year 2
Metrics
Since May 2022, the European Medicines Agency (EMA) has published a monthly report on key performance indicators related to implementation of the Regulation (link). Information from the most recent (25 January 2024) report is summarised below.
Overall, 3,339 clinical trial applications were submitted to the Clinical Trials Information System (CTIS) between the launch of the portal on 31 January 2022 and 31 December 2023, of which 2,473 were initial clinical trial applications, 625 were trials transitioning from the EU CTD, and 241 were resubmissions. The monthly submission rate is shown in the figure below.
Monthly Submissions of Clinical Trial Applications in CTIS
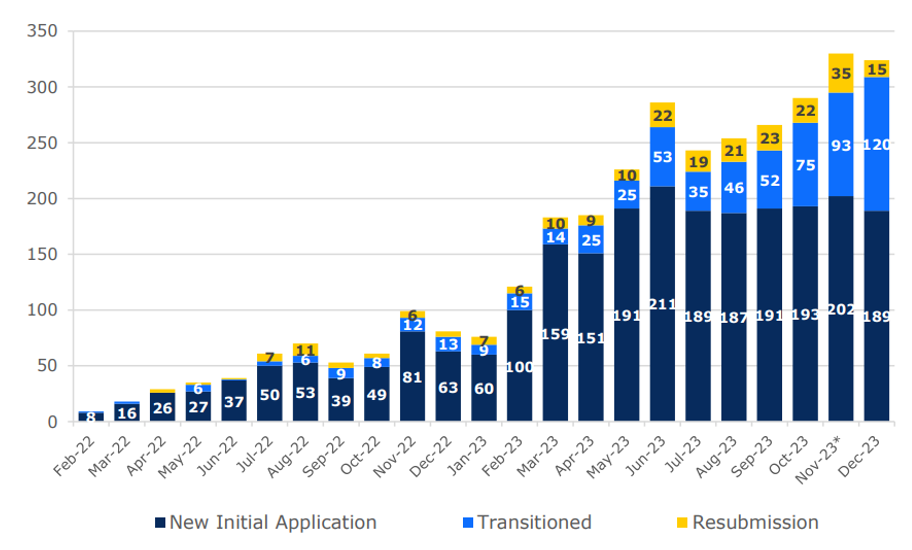
*Source: European Medicines Agency, ‘Monitoring the European clinical trials environment. A deliverable of the ACT EU Priority Action 2’, EMA/37858/2024, 25 January 2024 *(link)
Of the 3,339 clinical trial applications through to 31 December 2023, 1,806 have been authorised, 236 withdrawn and 183 lapsed, with most of the rest still under evaluation.
Of the 1,806 authorised trials, 1,042 (58%) were sponsored by commercial (including pharmaceutical company) sponsors and 764 (42%) by non-commercial (including academic) sponsors.
See the figure below for the average monthly time to decision for new applications.
Average Time from Submission of Initial Clinical Trial Application to Decision
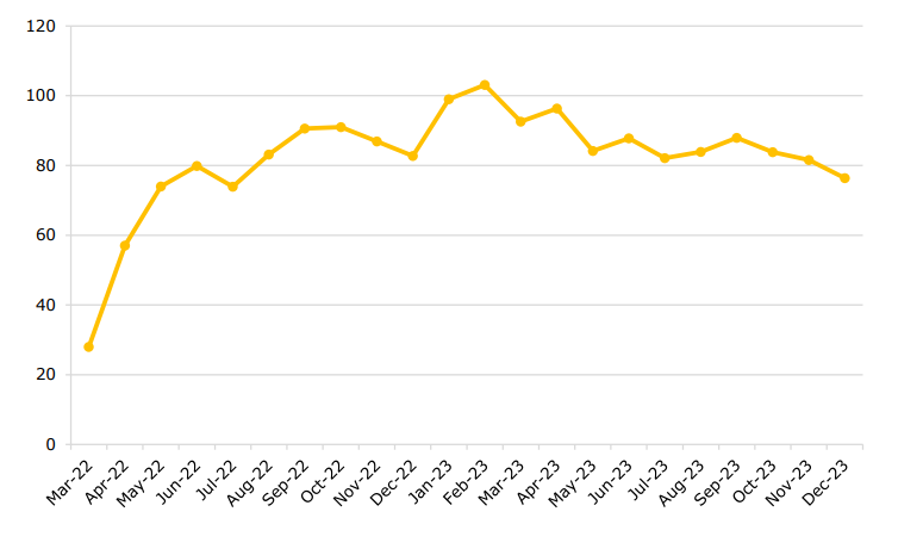
Source: European Medicines Agency, ‘Monitoring the European clinical trials environment. A deliverable of the ACT EU Priority Action 2’, EMA/37858/2024, 25 January 2024 (link)
As of 9 February 2024, information on 200 trials with a decision has been published on the CTIS portal. The sponsors of these trials would have opted not to request deferral of publication during the application process.
Revised Transparency Rules
The EMA management board adopted revised transparency rules (link) for the publication of information on clinical trials submitted through CTIS on 5 October 2023, following a public consultation held in May and June 2023. The aim of the new rules is to make CTIS less complex, more efficient, more user friendly, and to preserve transparency. Full implementation of the new transparency rules in CTIS is expected to occur in or after the second quarter (2Q) of 2024. (Update: Implementation will occur on 18 June 2024.)
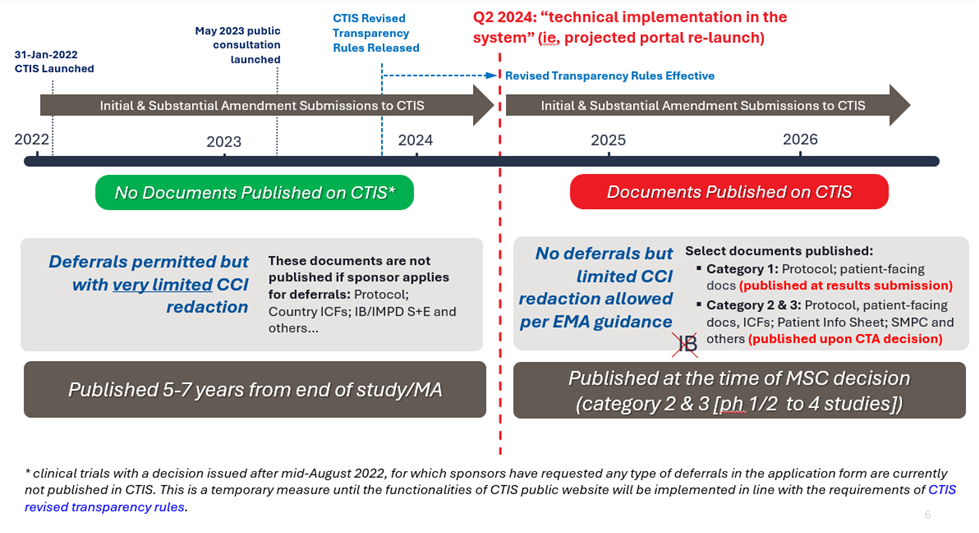
See the following figure for an overview of the history and the future of publication rules under the EU CTR. With the new rules, the deferral mechanism is removed and publication several years after the end of a trial no longer applies; however, documents for such deferred trials that are already in CTIS will not be published unless the trial is subject to modification after rule implementation (see further below).
Evolution of Publication Rules Under the EU CTR
The revised transparency rules introduce several noteworthy changes that include the following:
• Removal of the deferral mechanism: The option to defer the publication of documents has been eliminated, ensuring earlier dissemination and enhancing real-time transparency.
• Early publication of key documents: The new rules mandate earlier publication of key documents, focusing on information most relevant to patients, researchers and the public.
• Redaction of protected personal data (PPD) and confidential commercial information (CCI): Redaction will be the only tool to protect PPD and CCI. Striking the right balance between transparency and protection of CCI requires careful consideration by sponsors.
• Reduced burden for CTIS users: Publication of fewer documents will reduce the workload for users engaged in the necessary redactions.
Publication of the trial in CTIS will be triggered by the first member state concerned issuing a decision on the trial. Once the decision on the application has been issued, the latest structured data and documents are subject to publication. All applications (i.e. initial applications, substantial modifications, and additions of new member states) are subject to the same publication rules. For nonsubstantial modifications, data and documents are subject to publication when submitted to CTIS.
Tip: Sponsors should ensure stakeholders across their organisation understand that Phase II, integrated Phase I/II, Phase III and Phase IV protocols and patient-facing documents will become publicly available at the time of decision on a trial application.
The publication of structured data fields in the clinical trial application is largely unchanged, except for dose and strength details for integrated Phase I/II trials (falling under Category 2), as well as Category 1 trials (adult and paediatric populations) that will not be published at the time of the decision, but rather at the time of publication of the final results. In some instances, depending on the trial phase, dose details may be considered CCI. In such instances, sponsors can include ‘dummy data’ in the related structured data fields of CTIS (link).
The timing of publication of the documents in scope of the new rules is summarised in the table below. Any document not listed in the table, such as the investigator’s brochure, scientific advice letters, assessment reports, corrective measure documents and interim summaries of results is out of scope for publication.
Timing of Publication of Clinical Trial Application Documents in CTIS
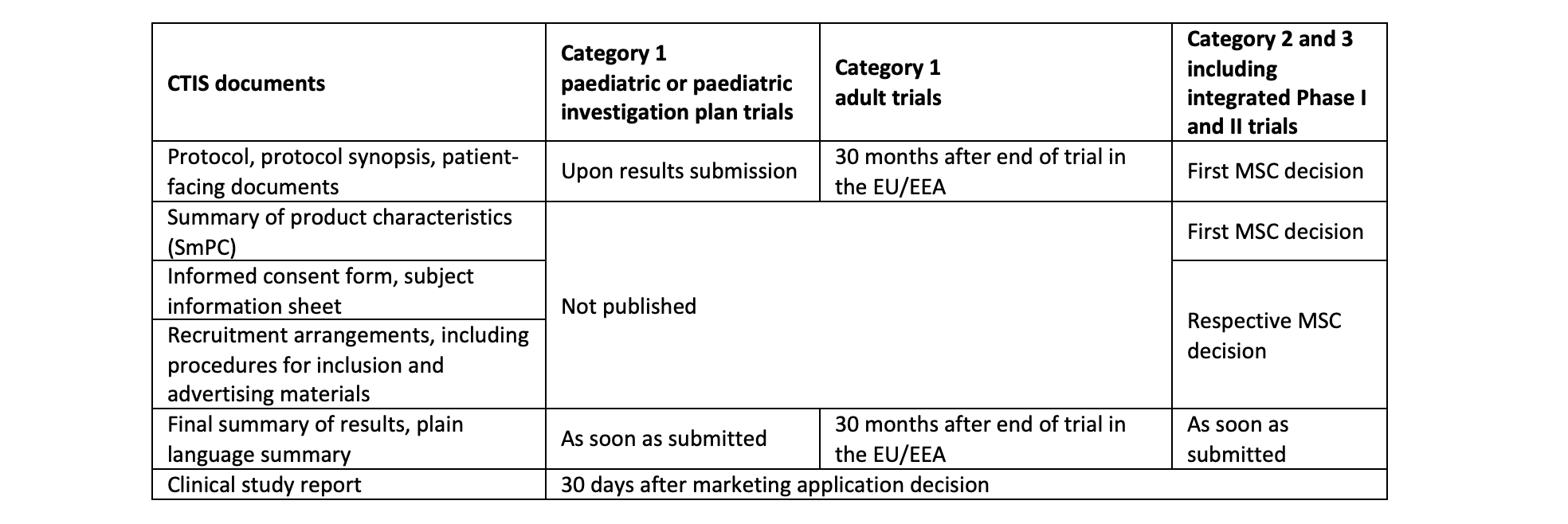
MSC = member state concerned
Source: European Medicines Agency, ‘Revised CTIS Transparency Rules’, Annex I. EMA/263067/2023, 5 October 2023 (link). See also: European Medicines Agency, CTIS Team, Data Analytics and Methods Taskforce ‘Revised CTIS Transparency Rules, Interim Period and Historical Trials: Quick Guide for Users’, 1 December 2023 (link)
Interim Period Prior to Implementation of New Rules
Sponsors are currently in an interim period prior to full implementation of the new transparency rules in CTIS. The EMA issued updated guidance on 31 January 2024 (link) that answered questions on how the publication of structured data and documents will be handled during this period, i.e. until the launch of the revamped CTIS portal in or after 2Q24. (Update: The launch will be on 18 June 2024.) Some of the key information was as follows:
Tip: Sponsors should review the latest guidance and determine what approach to take during the interim period until CTIS is updated to support the new transparency rules.
• For new clinical trial applications submitted during the interim period, documents in scope of the revised transparency rules should be redacted and uploaded in the slot ‘for publication’ in CTIS.
• For documents that are out of scope of the revised transparency rules, a placeholder page (example provided in the guidance) could be uploaded in the slot ‘for publication’.
• An unredacted version of the documents to be assessed must be provided in the slot ‘not for publication’ for evaluation by the member states.
• Trials submitted in CTIS prior to implementation of the technical changes are called historical trials. For such trials, structured data will be published for all trial categories as per the revised rules, while submitted documents will not be published.
• For transition trial applications, sponsors must submit a redacted version of the protocol and informed consent form(s) for publication.
• For patient-facing documents (e.g. patient questionnaires) for which written agreements establish that public disclosure is prohibited, the sponsor can upload a placeholder document in the ‘for-publication’ slot with a justification for not disclosing the document.
Tip: In-scope documents (protocol, patient-facing documents, etc.) for historical trials will be subject to publication after technical implementation of the rules whenever there is a modification to the trial.
The primary guiding application of the new rules is prompt publication of mandatory documents and structured data in CTIS. Therefore, while still allowed, the EMA advises against requesting deferral of publication for new clinical trial applications during the interim period. The EMA preference is for CCI in in-scope documents to be redacted to allow publication of the document with the decision date. Therefore, in the absence of a deferral, structured data and documents in scope under the new rules will be published. This includes trials in all phases, including Phase I.
Outlook for Year 3
Year 3 will be the final year for sponsors to transition trials that will be ongoing after 30 January 2025 to CTIS. Regardless of expedited procedures for transitioning trials to the EU CTR, sponsors should prioritise this work during the first half of 2024 to ensure adequate time to prepare documents for disclosure and permit member state authorisation by the 30 January 2025 deadline. Additionally, sponsors must prepare for the expected update of CTIS that will support implementation of the revised transparency rules.
In Year 3, sponsors will be monitoring the following regarding disclosure and transparency under the Regulation:
• Confirmation of the exact timing of implementation of the revised transparency rules and the date on which CTIS will be upgraded. (Update: Implementation will be on 18 June 2024.)
• Additional guidance on implementation of the revised transparency rules and supportive CTIS functionality
• Member state feedback on initial and transition applications during the interim period in which deferral is still being requested to protect CCI in structured data and documents (including for Phase I trials) that will be immediately published unless deferral is used
• Clarity on how to protect CCI in documents for deferred historical trials that will be subject to immediate publication following implementation of the new rules after a nonsubstantial modification or addition of a new member state to the trial
• Feedback on the use of dummy structural data to protect dosage information
• Information on the clinical study report disclosure process in CTIS and how it will align with disclosure of the same reports under the EMA Clinical Data Publication policy
• Additional information on expectations for submission of technical study results for both intermediate and final data analyses.
Authored by the PHUSE EU CTR Implementation Working Group Project.